Regenerative Medicine
LifeMap Discovery® as a Novel Database of Cell Therapies
LifeMap Discovery provides information related to cell-based therapies, which aim to apply stem, progenitor or primary cells towards treatment of degenerative diseases.
- A concentrated source of information on cell therapies spanning the different stages of development: including research, pre-clinical, clinical and marketed phases, being pursued by both non-profit organizations (NPO) and industries.
- The provided information has been manually curated from multiple literature sources, such as: scientific publications, press releases, patent applications and clinical trials registries.
- The comprehensive information presented for each cell therapy includes: an overview of the therapy, a list of therapeutic cells utilized in the specific treatment, mode and regimen of cell delivery, mechanism of action, formulation, in vitro data, animal models, preclinical data and related clinical trials.
Regenerative Medicine
Regenerative Medicine is the field of science aimed at repairing the structure and function of diseased or damaged organs and tissues, previously considered irreparable. Currently, the vast majority of existing treatments for chronic degenerative diseases is palliative or only delay disease progression or the onset of complications associated with the underlying illness. In some cases, organ transplantation can be performed (e.g., kidney, heart, liver); however this option is typically limited by the shortage of available donor organs. In addition, transplantation procedures introduce new sources of morbidity, such as those associated with the immunosuppressive treatment required following organ transplantation. Regenerative medicine holds the promise of repairing the faulty mechanisms underlying disease initiation and/or progression and of eliminating the need for donor organs and subsequent immunosuppressive treatment.
Regenerative medicine encompasses several main biomedical approaches, targeting the root causes of diseases, such as:
- Administration of biologically active molecules to induce regeneration of the injured organ/tissue.
- Tissue engineering of artificial organs/tissues, which involves in vitro generation of functional replacement tissue for clinical use. Cells are seeded on 3-D scaffolds capable of supporting tissue formation. Artificial organs (e.g., lung, liver, kidney, heart), comprised of both synthetic and cellular components, can be transplanted to the patients to functionally support or replace damaged organs. Tissue engineering uses scaffolds impregnated with growth factors that provide support to the growing cells inside the patient’s body. Researchers are working on developing natural scaffolds that will encourage healthy tissue growth, avoiding the formation of scar tissue.
- Cell therapies deliver stem/progenitor, primary and/or genetically modified cells, to facilitate regeneration of damaged cells within an organ/tissue.
As the first approach lies beyond the scope of this database, further details of the cell therapy approach, including, to some extent, tissue engineering, are provided below.
Cell Therapy
A cell therapy is a treatment that utilizes primary cells, stem/progenitor cells, or stem cell differentiation derivatives to replace or repair damaged organs/tissues. The cells can be intravenously injected, directly transplanted into the damaged tissue, or recruited from the patient’s own tissues to induce self-repair.
Stem cells are the most favorable cell source for cell therapy, due to their inherent self-renewal properties and differentiation potential. Stem cells have the plasticity to potentially differentiate into any type of tissue cells. Stem cells can migrate to injured areas within the body and differentiate into the cell types of the newly formed tissue, whereupon, they can replace the damaged ones, and/or secrete trophic factors that are required for tissue repair.
Cell therapies have several potential mechanisms of action:
- Replacement of injured or diseased cells and/or tissue, by the injected cell engraftment into the damaged tissue
- Stimulation of endogenous tissue self-healing processes
- Delivery of genetic or molecular therapies to the targets, by means of genetically modified cells
Cell therapy has been studied for multiple degenerative disorders and promising results from preclinical studies and clinical trials have been described for some.
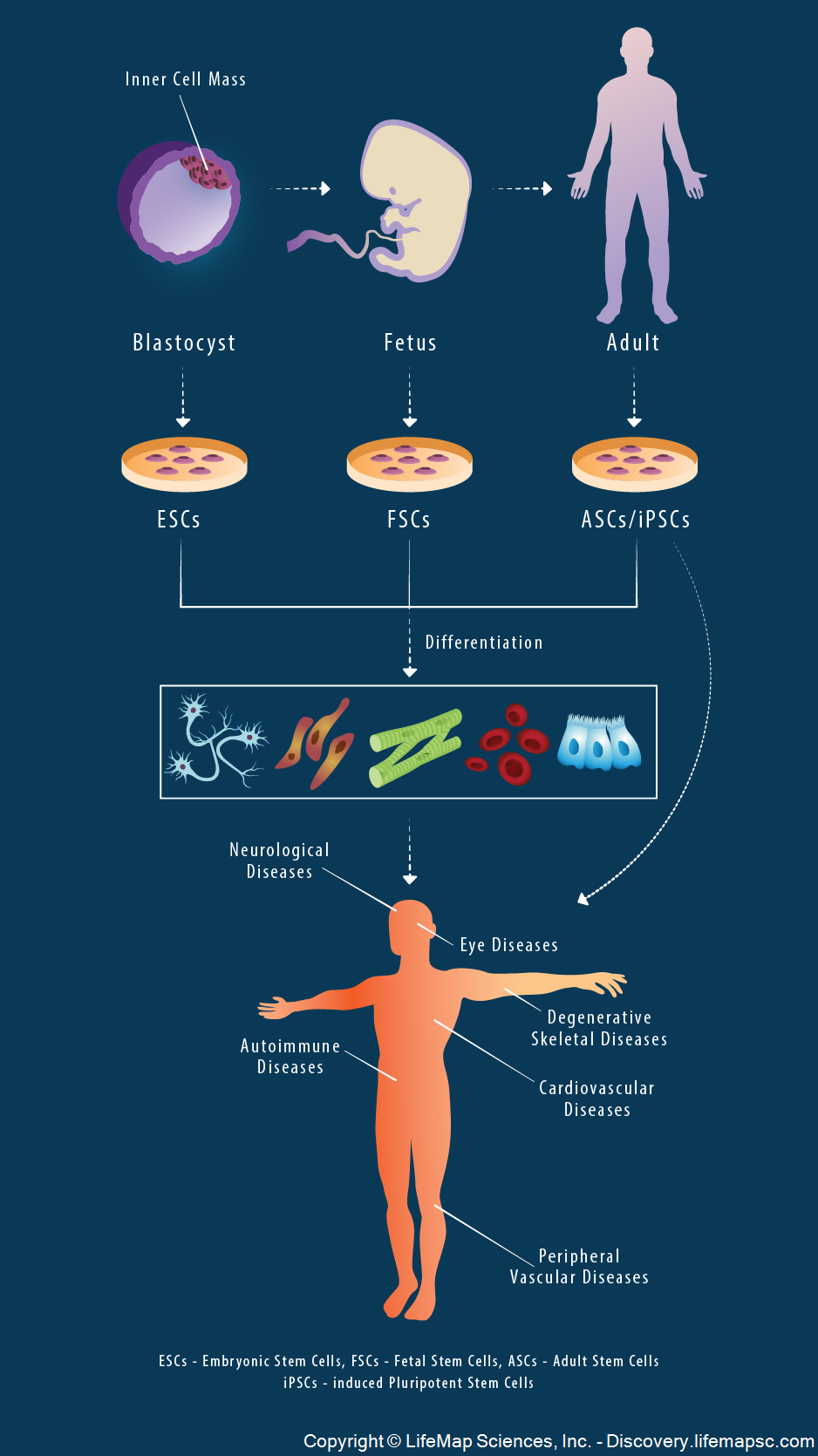
|
Numerous cell sources can be utilized for cell therapies: embryonic stem cells (ESCs) isolated from the inner cell mass of the blastocyst, fetal stem/progenitor cells (FSCs) isolated from fetal tissues, adult stem/progenitor cells (ASCs) or primary cells derived from adult tissues, and induced pluripotent stem cells (iPSCs) reprogrammed from somatic cells under specific conditions. Following isolation, any of these cell types can be cultured and expanded, and potentially differentiated or genetically manipulated to obtain the desired cell type. The resulting cells can be reintroduced to the patient's body to treat neurological, cardiovascular, degenerative skeletal and other diseases. |
Cell Therapy - General Considerations
Therapeutic Cell Types
Several cell types bear the features necessary for treatment of specific diseases, including: embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), fetal/adult stem/progenitor cells and primary cells. Feasibility and efficacy of each of the cell type options should be evaluated and compared.
Sources of Therapeutic Cell Transplants
- Autologous cells harvested from the patient, expanded ex vivo and then introduced back into the patient's body. Although autologous cell transplantation circumvents the need for immunosuppressive treatment, it is not always available or most effective.
- Allogeneic cells obtained from a donor organism of the same species. Allogeneic cell transplantation enables the use of "younger" cells, from fetal origins (e.g., umbilical cord blood, placenta) or healthy donors, which are considered to be effective due to higher regenerative potential and plasticity. In some cases, the nature of the medical condition will dictate the preference for autologous versus allogeneic therapy. For instance, during emergency care, allogeneic therapy is usually the only relevant option due to the time constraints of autologous cell expansion. Similarly, some conditions contraindicate autologous cell harvest.
- Xenogeneic cells obtained from a donor organism of an unrelated species. Xenogeneic cell transplantation is used when no human cells suitable for transplantation are available.
Immunological Considerations
Immune rejection occurs when recipient recognizes the transplant as a foreign entity and his immune system responds to eliminate it. Immune rejection is expected following allogeneic cell transplantations. Possible solutions can be provided by use of MSCs, which are known for their immunomodulatory properties, iPSCs, which are derived from the patient's own tissue, and of course autologous cell sources.
Cell Therapy Approaches
Cell Transplantation
Embryonic stem cells (ESCs) are derived from the inner cell mass of the blastocyst. They have unlimited potential to produce the specialized cells of the body, which introduces enormous possibilities for disease research and new therapies. Current challenges facing ESCs research include ethical considerations and the development of robust efficient differentiation protocols to prevent teratoma formation upon transplantation into the patient's body.
A number of clinical trials utilizing ESC-derived therapeutic cells have recently been initiated. A trial being conducted by Advanced Cell Technology is testing the effectiveness of MA09-hRPE, human ESCs terminally differentiated into retinal pigment epithelial (RPE) cells, in treating degenerative macular diseases. GRNOPC1, hESC-derived oligodendrocyte progenitor cells, have been assessed by Geron for treatment of spinal cord injury.
iPSC-based cell therapy enables to advance personalized medicine by utilizing autologous cells. Somatic cells from the patient are harvested and reprogrammed to produce a supply of clinically compliant pluripotent cells ready for use at any time later in life. Various groups have been working on two major hurdles associated with application of iPSC-based therapies: cost reduction of iPSCs development and development of clinical-grade processes for generation of iPSC lines.
Commercial entities offer personalized iPSC banking services, e.g., private cord blood banks, which store individual samples for future use. However, the long-term genetic stability of this cell type remains to be determined, so as to avoid tumor formation following therapeutic cell transplantations.
The applicability of iPSCs in cell therapies is currently under investigation. A pilot study assessing the safety and feasibility of the transplantation of autologous iPSC-derived retinal pigment epithelium (RPE) cell sheets in patients with exudative (wet-type) age-related macular degeneration was launched in Japan in August 2013.
Adult stem/progenitor cells are present in many organs and tissues, e.g., bone marrow, teeth, heart, gut, kidney and liver, and remain quiescent for long period of time until activated by a disease or injury trigger. In many cases of disease/injury, these tissues are maintained and repaired by the tissue-specific stem cells, which typically generate the cell types of the tissue in which they reside.
Examples of some of the most widely studied adult and fetal stem cells currently investigated in the clinical trials:
Bone marrow derived - hematopoietic stem cells (HSCs) were the first stem cells to be identified and to reach the clinic. These cells migrate to the bone marrow, where they self-renew and rebuild the entire blood system. Bone marrow transplantation is the only stem cell-based therapy currently accepted for clinical application. HSCs have been successfully used in cancer treatment, have shown promising results in treating autoimmune diseases and succeeded as additive treatment in patients with transplanted organs. LifeMap Discovery presents applications in different stages of clinical development, using HSCs for treatment of numerous diseases.
Mesenchymal stem cells (MSCs) are multipotent stromal cells, which are being actively assessed in cell-based therapy clinical trials. MSCs have the capacity to promote angiogenesis, differentiate and they possess immunomodulatory properties. MSCs have been widely tested in preclinical studies. Clinical trials have shown promising results for MSCs when applied to treat numerous diseases, such as cardiovascular diseases, brain and spinal cord injury, stroke, diabetes, cartilage and bone injury, Crohn’s disease and graft versus host disease (GvHD). In addition, the immunomodulatory effects of MSCs have been demonstrated in several conditions characterized by inflammation. However, a number of late-stage clinical trials have failed to meet primary endpoints and the fate of MSCs following systemic infusion is largely unknown.
MSCs can be isolated from different tissues, such as bone marrow, peripheral blood, placenta, umbilical cord and adipose. The efficacy of treatment with MSCs sourced from different tissues and cell availability from each source must be tested for each disorder. In some cases, no difference in efficacy of a specific MSC source was found. More in-depth studies are required to determine if certain MSC sources are more beneficial in specific diseases and if their therapeutic effectiveness and safety profiles are similar.
Bone marrow-derived mononuclear cells (BM-MNCs), a cell population comprised of hematopoietic progenitor cells, lymphoid cells (lymphocytes, plasmatic cells), monocytes, and macrophages. BM-MNCs are another cell population under extensive evaluation in clinical trials for treatment of cardiovascular diseases and for peripheral vascular disease, including critical limb ischemia, as well as for amyotrophic lateral sclerosis(ALS), stroke, obstructive pulmonary disease and more.
Primary cells In some cases, tissue-specific specialized primary cells can be used for cell therapy. However, this option is limited by the technical difficulties in obtaining and expanding the required cell type.
For example, administration of hepatocytes toward treatment of liver diseases is being tested. Primary human hepatocytes can be isolated from non-transplantable or fetal livers, or liver resections. The isolated primary hepatocytes have limited proliferation potential and their quality and metabolic/functional activity vary.
Engineered Tissue Transplantation
Tissue engineering involves the use of a combination of cells, engineering materials, and biochemical factors to improve or replace biological functions. LifeMap Discovery describes several transplantable tissue engineered cell sheets. Cell sheet techniques have been developed to prepare biological grafts, which have been applied, in the laboratory and clinic, to several diseased organs, such as heart, eye, kidney and skin.
Bioengineered Skin Substitutes
Reconstruction of tissue-engineered skin featuring both the epidermis and the dermis is the ultimate goal for improvement of healing quality and avoidance of scar formation, arising after extensive burns. Skin substitutes involve different cell composites, such as: fibroblasts, keratinocytes, epithelial cells and/or MSCs transplanted on membranes or with supportive matrix.
Cell Sheet Engineering
Cell sheet-based therapy allows tissue reconstruction from cells grown on a sheet. The cell sheet engineering techniques have been developed in order to avoid the limitations of tissue reconstruction using biodegradable scaffolds or single-cell suspension injections. This approach has been reported to enhance the outcome of cell transplantation, due to increased cell retention in the site of transplantation. In cardiac cell therapy research, increased cell retention was demonstrated following MSC sheet implantation. More extensive paracrine effects and subsequent cardiac function recovery were achieved by MSC sheet therapy. Further development of this approach towards clinical application is required.
Limbal epithelial stem cells sheets cultured on amniotic membranes for treatment of limbal stem cell deficiency (LSCD). LSCD is caused by the dysfunction of the stem cells within the limbal epithelium, leading to failure of corneal epithelial regeneration. As a result, re‐epithelialization by the conjunctival epithelial cells, accompanied by chronic inflammation, stromal scarring, neovascularization, and persistent epithelial defects are observed. Human amniotic membrane (AM), the innermost layer of the placenta is the most common substrate used for both in vitro cultures and as a vehicle for transfer of cells during transplantation, due to its structural similarities to the ocular surface, as well as its biological and functional properties.
Cell Therapy Modeling in LifeMap Discovery
LifeMap Discovery provides information on cell-based therapeutic products under development or marketed by either non-profit organizations (NPO) or by industry. Each cell-based treatment approach is presented as a potential therapy for a specific disease or a disease category (e.g.: cardiovascular diseases, metabolic diseases, etc.), studied in animal models or in humans. The provided information has been manually curated and can be searched and sorted by various criteria: disease, affected organ/tissue, product specifications, sponsorship or phase of development.
Additional information included in each cell therapy cards includes:
- Product description
- Mode and regimen of cell delivery
- Mechanism of action and formulation
- Related diseases, including comprehensive information about each disease, with links to MalaCards, the Human Disease Compendium.
- Description of the therapeutic cells utilized in the specific treatment, including isolation procedures and growth conditions, and gene expression profile.
- Research information including in vitro data, animal models and preclinical data
- A comprehensive listing of clinical trials evaluating the therapy
- References and publications
- Relevant links to press releases, patents and more.
Cell Therapy Targeted Diseases
Cell therapy is a novel technology which bears the potential to treat a wide range of human degenerative diseases. Intractable diseases, disorders, and injuries are characterized by cell death or aberrant cellular function. Cell transplantation can replace diseased or lost tissue, thereby providing restorative therapy for these conditions.
LifeMap Discovery presents cell therapies being assessed for multiple diseases. Most of the current applications focus on approximately 20 most explored diseases that can maximally benefit from cell-based treatment:
Disease List
Each Disease card in LifeMap Discovery includes a brief disease description, a detailed description of the current cell therapy approaches, list of cell therapies affiliated with the specific disease and relevant references. All the data are manually curated from up-to-date publications and from the clinicaltrial.gov website.
Clinical trial search at ClinicalTrials.gov
used to search stem cell-related
clinical trials at ClinicalTrials.gov:




